You may be infusing more lock solution than you think into your animals.
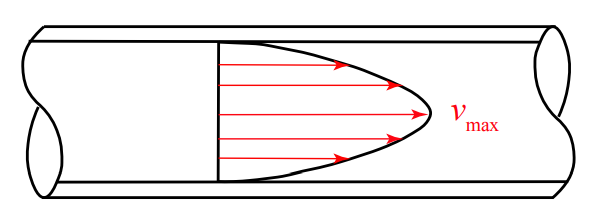
The flow of fluids in the small tubes used for rodent catheters is almost always governed by laminar flow. In laminar flow, fluid at the center of the tube moves faster than fluid at the edge which is held up by contact with the tube wall.
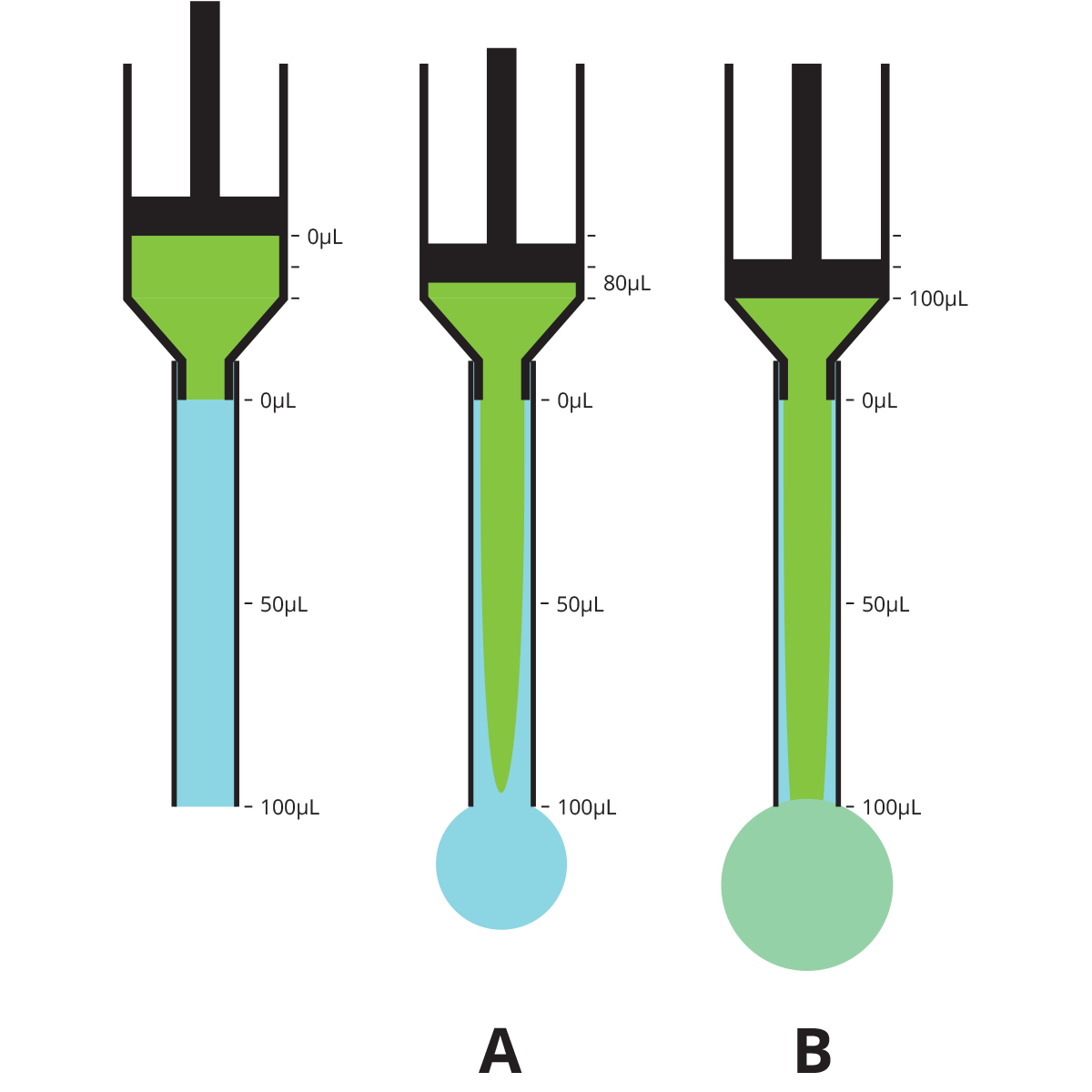
Filling Catheters With Lock Solution
Because fluids do not move with a flat front edge, if you have a implanted catheter with a dead volume of 100µL and you infuse exactly 100µL of lock solution into it, you may think you are just filling the catheter, but in fact the tip of the cone of lock solution will exit the catheter and enter the animal’s bloodstream, while the catheter will still have some of whatever fluid was originally in the tube, often blood, near the edges.
We conducted bench top tests* to determine how viscous heparin-50% glycerol lock solution flows in a typical 3Fr rat catheter prefilled with saline with a dead volume of 100µL (approximately 30cm long).
| Volume infused when lock solution reaches tip of catheter (A) | 80µL (80% of dead volume) |
| Volume of lock solution infused into animal when filled with exactly 100µL (B) | 4µL (4% of dead volume) |
| Volume infused to completely fill catheter with lock solution | 170µL (1.7x dead volume) |
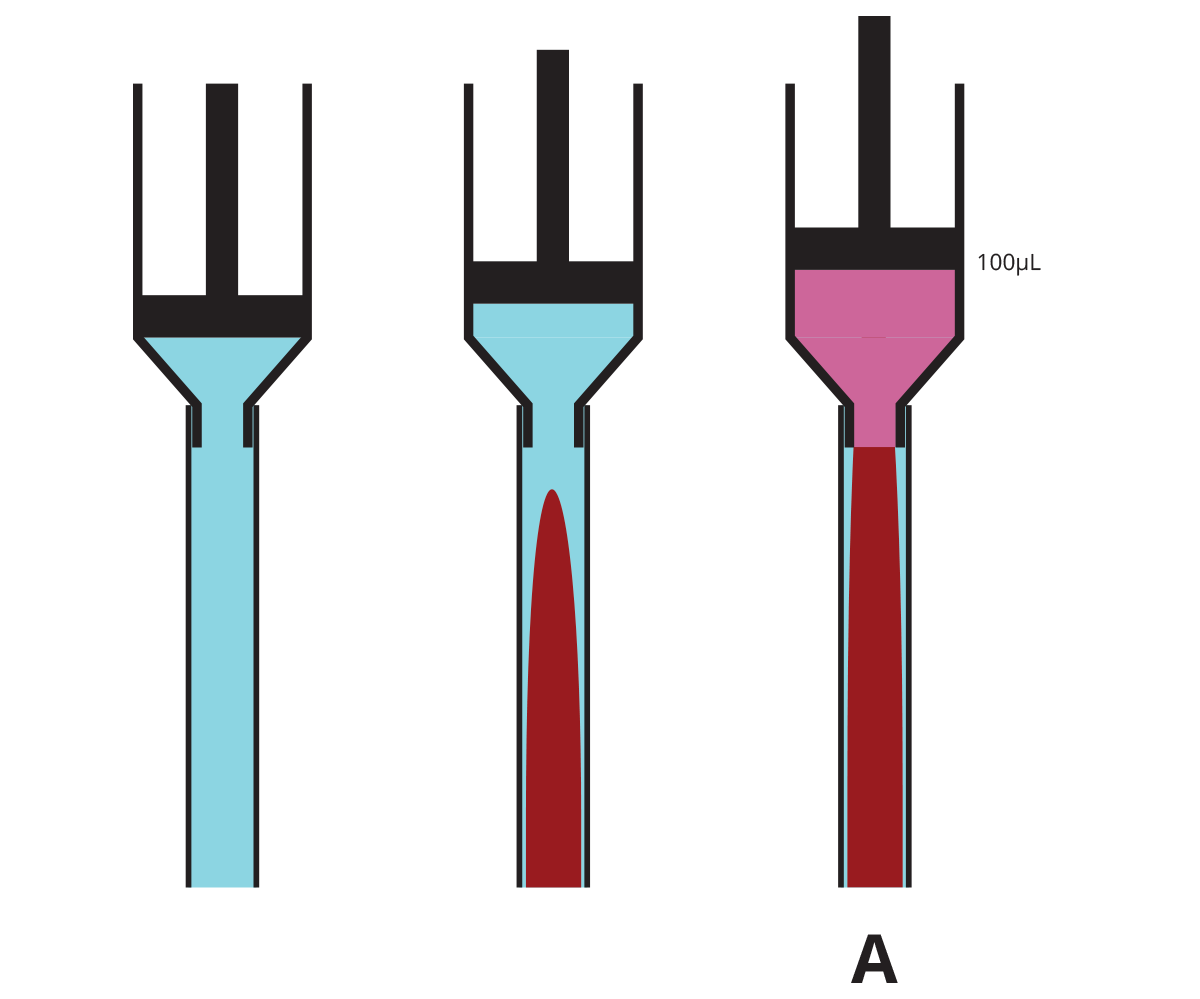
Clearing Catheters of Lock Solution
When you pull back on a syringe to remove lock solution from a catheter, laminar flow may also cause unexpected results. Blood will travel up the center of the tube, leaving lock solution on the edges.
We conducted bench top tests* to determine how saline flows through a catheter with 100µL dead volume that is prefilled with heparin-50% glycerol lock solution.
| Volume of hep-glycerol remaining in catheter after 100µL pulled out (A) | 8µL (8% of dead volume) |
| Volume required to be pulled out to clear all hep-glycerol from catheter | 200µL (2x dead volume) |
Implications
Unless you are precisely filling the catheter with no more than 80% of the dead volume, you should assume that some of the lock solution you are using will be infused into the animal.
Even the best tubing extruders cannot hold tolerances better than ±.002in, which translates into ±15% volume variability in 3Fr catheters and ±30% in 2Fr catheters. So adding in the laminar flow effect, if you are using a nominal rather than a measured value for the dead volume you can assume that 20% or more of your lock solution could be infused into the animal when you are filling the catheter.
Unless you are pulling out at least double the dead volume of the catheter when removing the lock solution, some will remain around the edges of tube and could be infused back into the animal in a later step.
Viscous glycerol or dextrose lock solutions with high concentrations of heparin (500 IU/ml) are a standard based on the “Comparison of Catheter Lock Solutions in Rats” publication by Dr. Luo et al. in 2000. However, the catheters in that experiment were externalized and sealed with a plug which was removed for access; the Vascular Access Button™, which permits aseptic access technique and keeps the catheter completely subcutaneous, was not available then. More recent publications have demonstrated excellent patency with low concentration heparin-saline as lock solution or even just saline itself. Given the likelihood that lock solutions will be infused into the animal, researchers and IACUCs should consider whether potentially harmful viscous high-concentration heparin lock solutions are safe and necessary.
*Bench top testing methods: fluids infused into Instech catheter tubing using syringe pump at 100µl/min. Leading edge of cone estimated visually with dye in infusate. Trailing edge determined using conductivity sensors on Instech ABS2. Certain volumes (4% in infusing, 8% on removing lock solution) estimated using volume formula for paraboloid.