Maintaining catheter patency throughout a laboratory animal infusion study is the primary goal of the surgeons that implant the catheters and the technicians that access them. There have been meaningful improvements in rodent catheter patency over the past 15 years with the development of systems that are closed from bacteria and keep the catheter tubing subcutaneous to avoid evaporation, as well as a greater realization of the importance of aseptic surgery, ideal tip position and positive pressure technique. However, despite these advances, as you can see from the chart below, the chance that your studies will be completely free from patency problems is low.
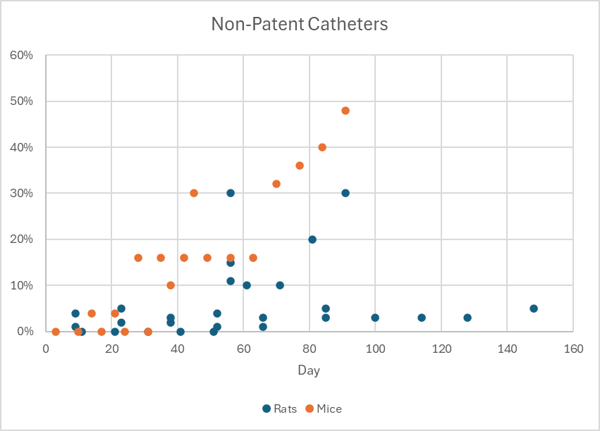
Percent of non-patent catheters by day from a collection of studies of rodent catheter patency. Only the “best” and “recommended” arms of the studies are included here.1
These studies only look at the patency of implanted catheters, so blockages and kinks in the lines outside of the animal will add to these numbers in practice.
If you are using a pump to deliver your compound, it’s critical that you understand how the pump will react when there is an occlusion and plan how you or your technicians will respond when it happens. If you are choosing new pumps, you need to know how to evaluate a particular pump’s performance with the types of occlusions you might actually experience in your studies. For example, clinical pumps are designed to detect the occlusions that occur in human medicine, not a mouse infusion study with flow rates measured in microliters per hour.
As we showed in our 2015 presentation at the Infusion Technology Organisation conference, there is a large variation in performance among the pumps that are commonly used for laboratory animal infusion. You cannot simply check a box on whether a pump has an occlusion alarm or not.
The Types of Occlusion Alarms
The method that a pump uses to sense an occlusion is the most important factor in its performance. Most pumps fall into four categories:
- None. The motors on older laboratory syringe pumps will simply turn until they reach their maximum force limit, and then they will stall. New Era, Chemyx and older Harvard Apparatus and KD Scientific pumps fall into this category. These pumps have maximum force specifications of 35-45lbs, which translates into 1,300-1,700PSI with a 1mL syringe. This is 10x the pressure at which most infusion sets will rupture.
- Force limit. Newer Harvard Apparatus pumps, such as the 11 Elite series, let you set a force limit, a percentage of the maximum force at which it will alarm and stop. The manual states this is to avoid breaking syringes, not to detect occlusions. The lowest you can set this limit is 20-30%; below that the pump does not have enough force for normal operation. 20% of the max force translates into 260PSI with a 1mL syringe, a pressure at which nearly all infusion sets will rupture.
- Motor current. A cousin of the force limit, some older clinical pumps monitor the current that the pump is drawing and try to interpret when a struggling motor might indicate an occlusion. Unlike a simple force limit, these pumps were designed to detect real occlusions that occur in hospitals, adjusting for the syringe that’s being used and perhaps looking for a change in current rather than just the absolute value. However, this is still an indirect method—friction and play in the mechanism makes this a noisy signal, particularly for small syringes. If the pump’s mechanical components are plastic, they will bend before the motor can sense the occlusion. The Baxter AS50 and its non-clinical replacement the SAI 3D fall into this category. In fact, the AS50 had an FDA recall in 2005 in part due to problems sensing occlusions.
- Force sensor. The most accurate method of detecting an occlusion is to measure the actual force on the syringe plunger with a sensor. Not only will this method detect occlusions more quickly than monitoring motor current, but it is less likely to have false alarms. The signal-to-noise ratio is much better. Modern clinical pumps such as Medfusion and the OrchesTA™ model 100 have force sensors, as does the new Instech model 400 pump for laboratory animal research.
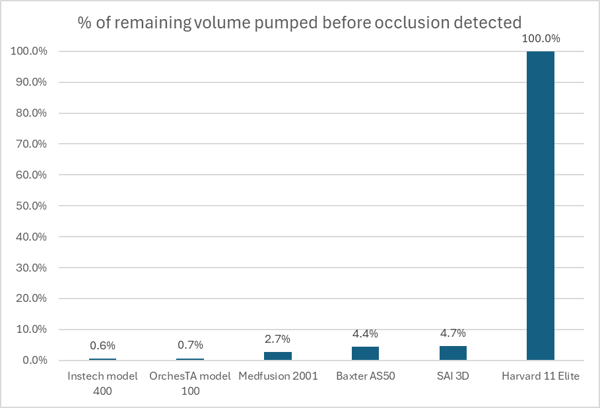
Performance of various pumps with an occlusion of a rat infusion set while pumping at 2mL/h over 1h with 3mL syringe. (The Harvard pump did not stop and ruptured the infusion set.)2
The data above shows that pumps that use force sensors to detect occlusions (Instech, OrchesTA™ and Medfusion) react more quickly than pumps that rely on motor current (Baxter and SAI), while the Harvard pump did not detect the occlusion at all.
Detecting Occlusions at Low Rates
The rat infusion study above is still within the range of human medicine, with 2mL delivered over 1hr. How do these pumps perform when delivering microliters, as is typical with mouse infusion and intrathecal dosing?
To answer that question we tested four pumps using the following parameters:
- A bolus of 20µL delivered over 4 minutes (a rate of 5µL/min or 0.3mL/h)
- BD 1mL syringe, free of air bubbles and primed using the pump to remove slack from the system.
- 90cm of PE/PVC tubing to a mouse VAB with a 10cm 2Fr polyurethane jugular vein catheter, similar to what might be used to deliver an intermittent dose to a mouse. Total dead volume is approximately 150µL.
- The output of the catheter is connected to a metal tube which empties into a small beaker on electronic balance with 0.1mg sensitivity which is connected to a computer to record the data, with precautions to minimize the effect of evaporation, all per ISO standard 7886-2.
- TRIAL 1: measure accuracy and flow profile of an unoccluded 4 min dose.
- TRIAL 2: occlude the 2Fr catheter with a hemostat and then start the bolus dose. Measure time to detect occlusion. Follow manufacturer’s recommendation to respond to occlusion on pump. Release the hemostat to free the occlusion (this would simulate a common scenario such as a positional occlusion that is resolved when the animal moves). Restart the pump. Measure dose accuracy and flow profile.
Results
The differences in occlusion detection between pumps with this 20µL bolus test are stark. The Instech model 400 pump, which was designed for these rates, detected the occlusion after about 40 seconds of the 4 minute dose - just 3µL was pumped into the 150µL dead volume of the tubing set. The OrchesTA™ and Baxter pumps, which were operating at the very lowest end of their intended range, alarmed after more than half of the dose had been pumped. The Harvard pump finished the dose with no indication of an occlusion; the entire 20µL volume was absorbed by expansion of the tubing set.
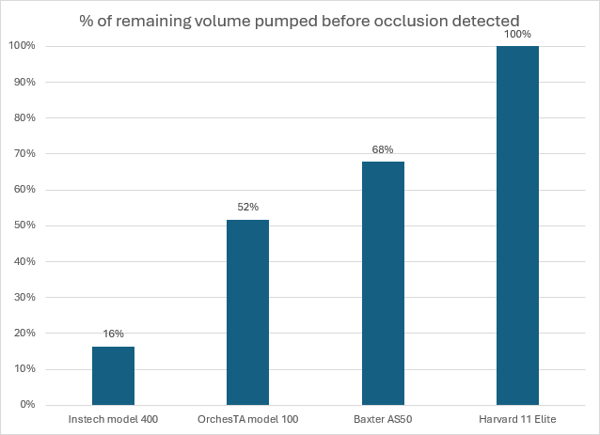
Performance of various pumps with an occlusion of a mouse infusion set while infusing a 20µL bolus.
All four pumps delivered the unoccluded dose accurately, within the ±5% specification for plastic syringes. Even the Baxter pump, which warns about accuracy with small doses in its specification of “±3% or .007in of travel, whichever is greater” was on the money in this test. Its problem was that 0.02mL was below the pump limit, so it changed the volume to 0.03mL automatically.
In the end, all three pumps with occlusion alarms managed to deliver the occluded dose relatively accurately, with 10% or less (1-2µL) lost in the process. However, the clinical pumps had much more of an adventure getting there, as you can see from the flow profiles in the details below, and if we were not simulating a positional occlusion their error would have been much greater. The Harvard pump failed to deliver the dose.
|
Target Volume |
TRIAL 1 (unoccluded) |
Error |
TRIAL 2 (occluded) |
Error |
|
|
Instech model 400 |
20µL |
19.4µL |
-3% |
18.7µL |
-7% |
|
OrchesTA™ model 100 |
20µL |
20.4µL |
2% |
18.9µL |
-6% |
|
Baxter AS50 |
30µL |
29.4µL |
-2% |
27.1µL |
-10% |
|
Harvard 11 Elite |
20µL |
19.4µL |
-3% |
0.0µL |
-100% |
Details by Pump
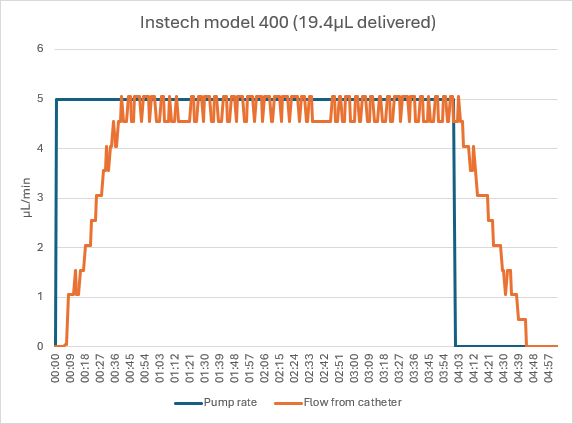
Instech Model 400
The Instech pump delivered the unoccluded dose in approximately 4.75 minutes, with a ramp up when the pump starts and the tubing expands, and down at the end of the dose as the tubing returns to its original shape. (The fluctuations are due to the 0.1mg resolution of the balance, not actual flow rate pulsatility.)
When dosing with an occluded catheter, the Instech pump sensed the occlusion after 39 seconds. As shown in the video below, the operator pressed the Release button until he could see that the pressure returned to normal; the pump counted back time and volume delivered based on the exact amount that the pump backed up. The hemostat was removed from the catheter and no stored volume was delivered to the animal. The operator pressed Resume and the pump delivered the dose as it had in the unoccluded case.
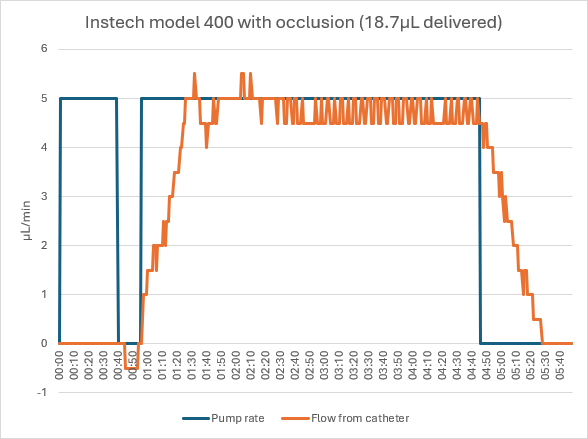
Responding to an occlusion with the Instech model 400 pump (video)
OrchesTA™ Model 100

The OrchesTA™ pump delivered the unoccluded 20µL bolus accurately and with a similar ramp up and down.
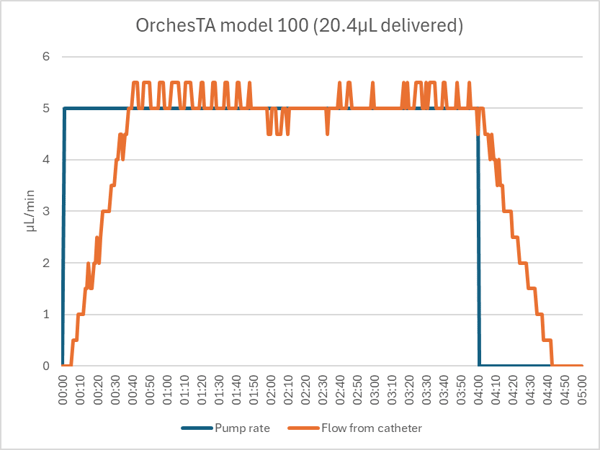
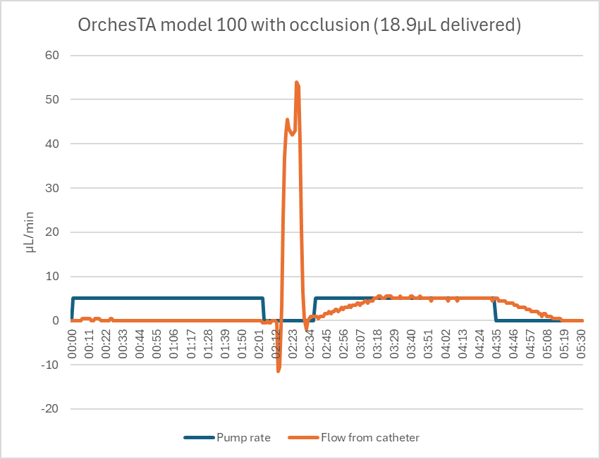
The OrchesTA™ pump detected the occluded catheter after 2:04 of the 4:00 dose. Despite a feature that automatically backs up the motor on occlusion, the pump did not fully release the pressure. When the hemostat was opened, a stored volume of 8.4µL was released (into the animal, if there had been one) in about 8 seconds. When the pump was restarted, it delivered the rest of the dose with the normal ramp up and down.
If the occlusion had been such that the line had to be opened, to flush the catheter for example, or if the pump was opened manually to relieve the pressure, then the stored volume would have been lost and only 10.5µL would have been delivered, a 48% dose error.
Baxter AS50

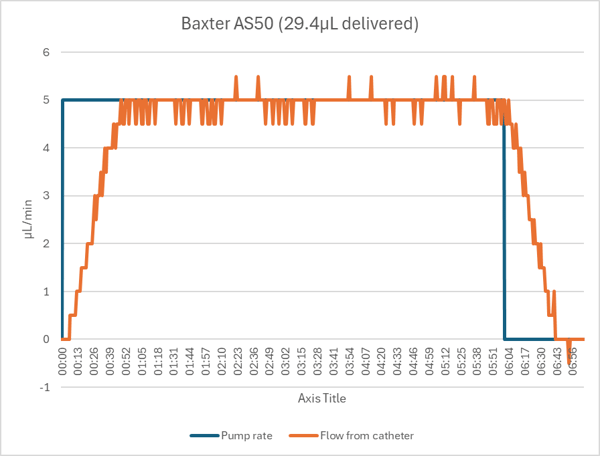
The Baxter pump gave a “PUMP LIMIT” error when we entered a target volume of 0.02mL. It automatically changed the value to 0.03mL, so we conducted the pump’s test with a 6 minute/30µL bolus instead. One minute into the infusion it gave a “<5MIN VOL LIM” alert, but the pump did not stop. Although it was clearly operating at the bottom end of its range, in the end it delivered the 30µL bolus accurately and with the normal ramp up and down due to tubing expansion.
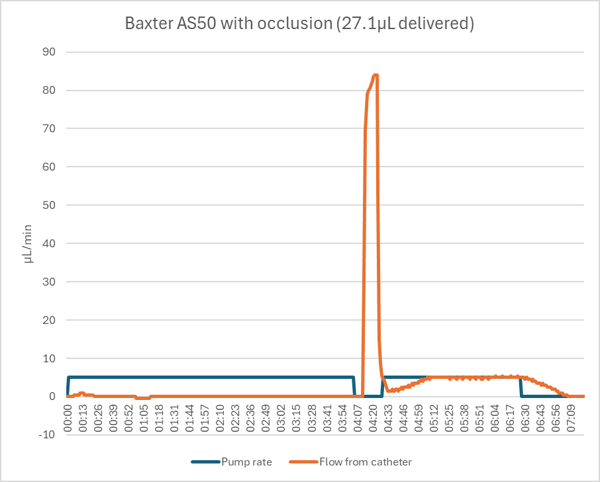
The Baxter AS50 did not detect the occluded catheter until 4.1 minutes of the 6 minute dose had passed. When the hemostat was opened a stored volume of approximately 17µL was released in about 10 seconds. When restarted, the pump continued with the remaining 1.9 minutes of the dose with the normal ramp up and down.
If the occlusion had been such that the line had to be opened or if the pump was opened manually to relieve the pressure, then the stored volume would have been lost and only 10.1µL would have been delivered, a 66% dose error.
Harvard Pump 11 Elite

The Harvard pump delivered the unoccluded bolus accurately and with the expected flow profile.
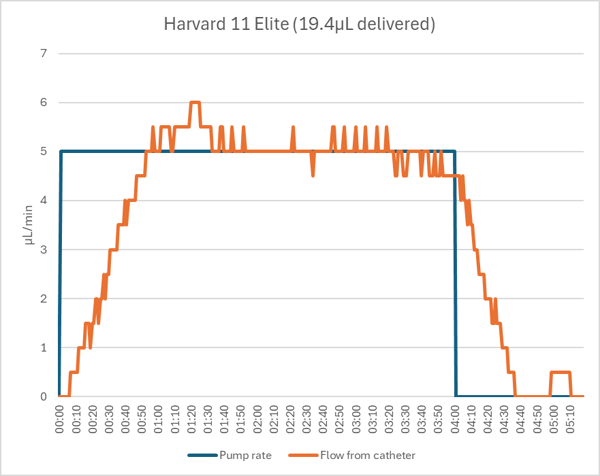
The Harvard pump did not detect the occlusion and continued to run for the full 4 minutes. The 20µL dose was trapped in the tubing, leading to a 100% dose error.
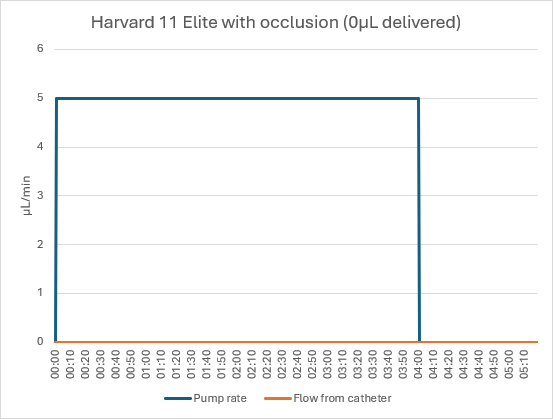
Conclusion
If you are infusing laboratory animals, you are nearly certain to experience an occlusion. If you are using an industrial pump with no occlusion alarm, you may not even notice that your dose failed. It could be stored in the tubing, which would then drip out if you disconnected the animal, or push the plunger backwards if you removed the syringe from the pump first. It could also leak out of a joint in your tubing set and drop into the bedding. Larger doses could rupture the tubing more dramatically, possibly disconnecting the catheter and releasing a dangerous bolus into the animal.
Clinical pumps have occlusion alarms, but they were designed for the flow rates used in human medicine. Their occlusion detection does not perform well at low flow rates, leading to delays in detection and sudden boluses released into the animal when the blockage is fixed.
The new Instech model 400 pump was designed for the flow rates and occlusions that are seen in laboratory animal research. The pump can detect occlusions with volumes of only a few microliters, and it lets you relieve the pressure intelligently using the pump so that you can avoid unintended boluses when the occlusion is fixed, and then continue to deliver an accurate dose as you originally planned.
References
1Rat studies: Harlan tox infusion study developments (2009) - VAH/PU arm, CRL comparison of catheter tip style (2022) - groups 1, 2 and 6, CRL refinements to IV infusion systems in rats (2019) - all data. Mouse studies: CRL patency of JV catheters in CD1 mice (2017) - VAB/7day flushing arm, CRL refinement for long-term repeated bolus injections in mice (2019) - all data.
22015 ITO presentation on impact of occlusions on dose accuracy, p11 backup data. Instech Model 400 tested under same conditions; occludes in an average of .26 min.